this post was submitted on 30 Jul 2023
73 points (95.1% liked)
Solarpunk
5496 readers
68 users here now
The space to discuss Solarpunk itself and Solarpunk related stuff that doesn't fit elsewhere.
Join our chat: Movim or XMPP client.
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
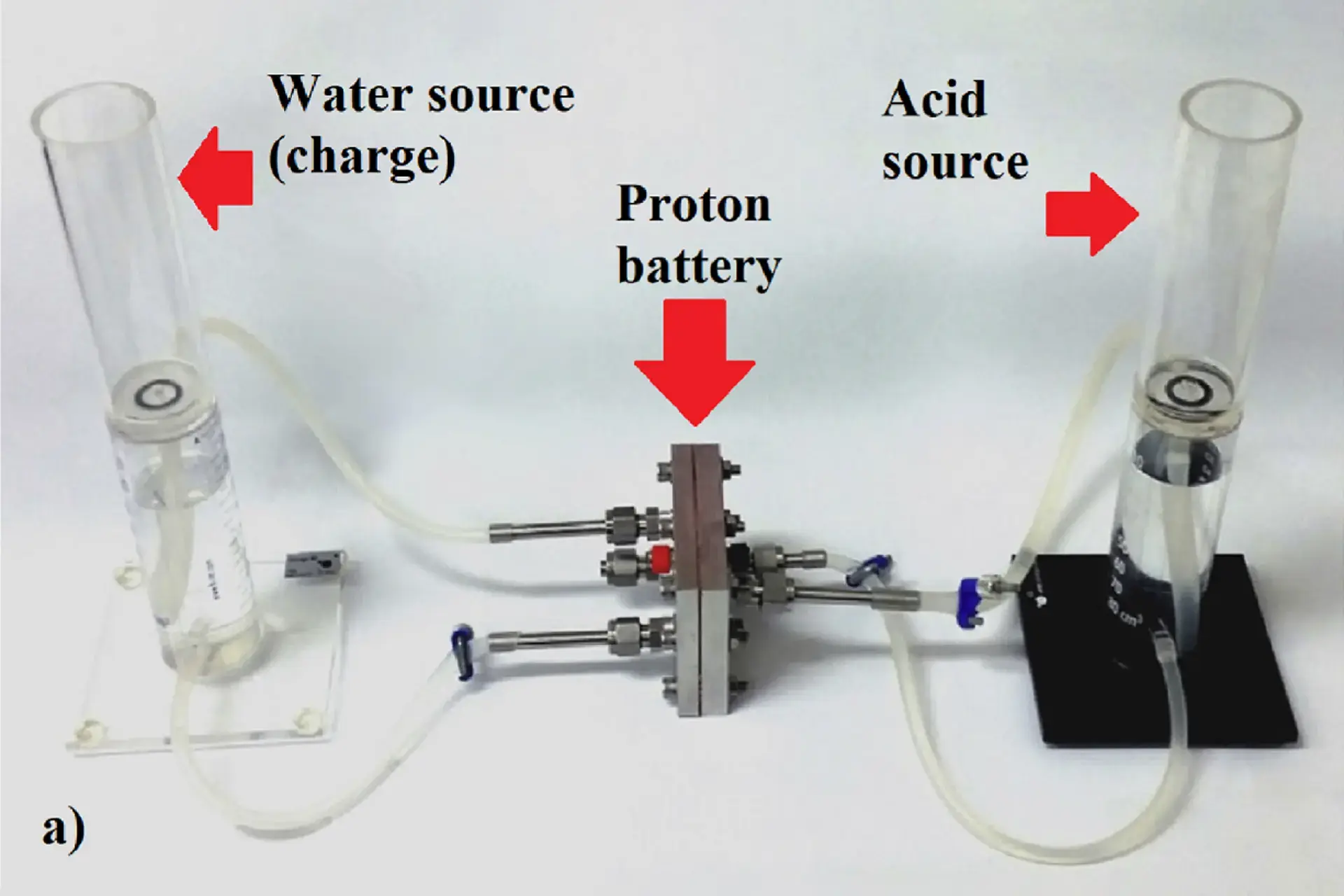
This is such a puzzle, thank you. :)
I checked Sci-Hub - no matches for "proton battery", neither for "hydrogen flow battery".
Falling back on chemistry - I recalled that "dissolving a concentrated acid in water should be done with care". It is exothermic, water may suddenly boil and splash acid all over the careless chemist.
By definition, acids are substances that can easily give protons (hydrogen ions) to other chemicals. A classic reaction would be acid + base = salt + water (acid gives the H and base gives the OH, so we get H2O), the other components of the acid and base form the salt.
If there is only water on the other side, I thought "the reaction is acid giving protons to water". Which acid? How many protons? Those questions might determine the amount of power available. And of course - how to control the reaction and extract electrical power? Browsing Wikipedia, I came across two pages: protonation and deprotonation and a sample reaction with sulphuric acid, but found no reference to electricity production, though potential / voltage is obviously available when ions are being created and transfered.
Then, finally I found the RMIT press release, and understood that acid is not a central participant of this reaction:
https://www.rmit.edu.au/news/all-news/2018/mar/all-power-to-the-proton
Some pickings:
There is a photo of the three scientists with a cell and multimeter, and the meter reads 1.1559 volts, so we know the cell voltage is low, but not impractically low. A link to a scientific article and a description of the cell follows:
"Technical feasibility of a proton battery with an activated carbon electrode"
(this implies that overcharging results in hydrogen formation, like in lead acid batteries - the solution is to have it vented typically)
(this leads to the question of oxygen availability on the other side, and how to ensure it's adequate - gases are a nuisance due to their low density, but water can dissolve only so much oxygen, and this could limit the power output or storage capacity of the cell, however, if one built a flow battery, a redundantly large mass of water could be used to supply oxygen - but I'd really like to know if they used gaseous or dissolved oxygen)
A summary of the scientific paper:
So, the acid was not a reaction participant, but a proton conductor.
If anyone has a copy of the paper, please share - it seems like it would be interesting. :)
DUDE Thank you!
This was super interesting to read.
Really gives me extra hope that this isn't another vaporware battery technology.
Perhaps the end of excessive lithium mining is in sight!
I'll take it with a grain of salt: